First Law of Thermodynamics
First Law of Thermodynamics: Overview
This topic covers concepts such as First Law of Thermodynamics and Mathematical statement of First Law of Thermodynamics.
Important Questions on First Law of Thermodynamics
When mol of a gas is heated at constant volume, temperature is raised from K. If heat supplied to the gas is J, then which statement is correct?
If is the change in enthalpy and the change in internal energy accompanying a gaseous reaction, then
A gas is enclosed in a cylinder with a piston. Weights are added to the piston, giving a total mass of . As a result, the gas is compressed and the weights are lowered . At the same time, of heat is evolved from the system. Calculate the change in internal energy of the system and report the answer in the nearest integer value
Find the change in the internal energy of a closed system, when denotes the amount of work done by the system and amount of heat is supplied to the system.
is mathematical expression for
State first law of thermodynamics. Justify its mathematical equation.
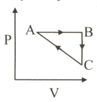
Consider an ideal gas which undergoes a cyclic process as below:
Then the Heat absorbed by the system during process CA is ____
Air separated from the atmosphere by a column of mercury of length is present in a narrow cylindrical soldered at one end. When the tube is placed horizontally the air occupies a volume . When it is set vertically with its open end upwards the volume of the air is . The atmospheric pressure during the experiment is . Find .
A cylinder of ideal gas is closed by a movable piston of area . When the gas is heated from to , the piston raises . The piston is then held in place and the gas is cooled back to . If is the heat added to the gas in the heating process and is the heat lost during cooling, then can be approximated as , where is an integer between (0 to 9). Find .
Given : Patm = 1 × 105 N/m2
g = 9.8 m/sec2
A system absorbs of heat and does of work. What is the net change in the internal energy of the system?
What will be the change in internal energy when of work is done on the system and of heat is given by the system?
In an adiabatic expansion of an ideal gas
Temperature of 4 moles of an ideal gas is raised from 300K to 350K. What is the value of for this process?
Ideal gas is contained in a thermally insulated and rigid container and it is heated through a resistance by passing a current of 1A for five minutes, then change in internal energy of the gas is
The molar heat capacity of water at constant pressure is . When 1KJ of heat is supplied to 100 g of water, which is free to expand the increase in temperature of water is
A heat engine absorbs heat at temperature and heat at temperature . Work done by the engine is This data
For a cyclic process, which of the following is not true?
A heat engine absorbs heat at temperature and heat at temperature . Work done by the engine is This data
The bond energy of is . It means that
Work Done in Thermodynamical Process, Law, Internal Energy & Heat Capacity. The internal energy when a system goes from state A to B is 40 kJ/mol. If the system goes from A to B by a reversible path and returns to state A by an irreversible path. What would be the net change in internal energy?